
H pylori testing and eradication for adults
• Patients with uncomplicated dyspepsia unresponsive to lifestyle change and antacids, following a single one month course of proton pump inhibitor (PPI),
without alarm symptoms.
• Patients with a history of gastric or duodenal ulcer or bleed, if they have not previously been tested.
• Before starting or taking NSAIDs, if there is a history of gastro-duodenal ulcers or bleeds. Note that HP and NSAIDs are independent risk factors for
peptic ulcers, so eradication will not remove all risk.
• Patients with unexplained iron-deficiency anaemia, after negative endoscopic investigation has excluded gastric and colonic malignancy, and
investigations have been carried out for other causes, including: cancer, idiopathic thrombocytopenic purpura, vitamin B12 deficiency.
Before stool antigen or urea breath testing for H pylori, patients should have stopped bismuth or PPI for at least 2 weeks; antibiotics for 4 weeks; or
results may be unreliable
• Patients with proven oesophagitis, or predominant symptoms of reflux, suggesting GORD (gastro-oesophageal reflux disease)
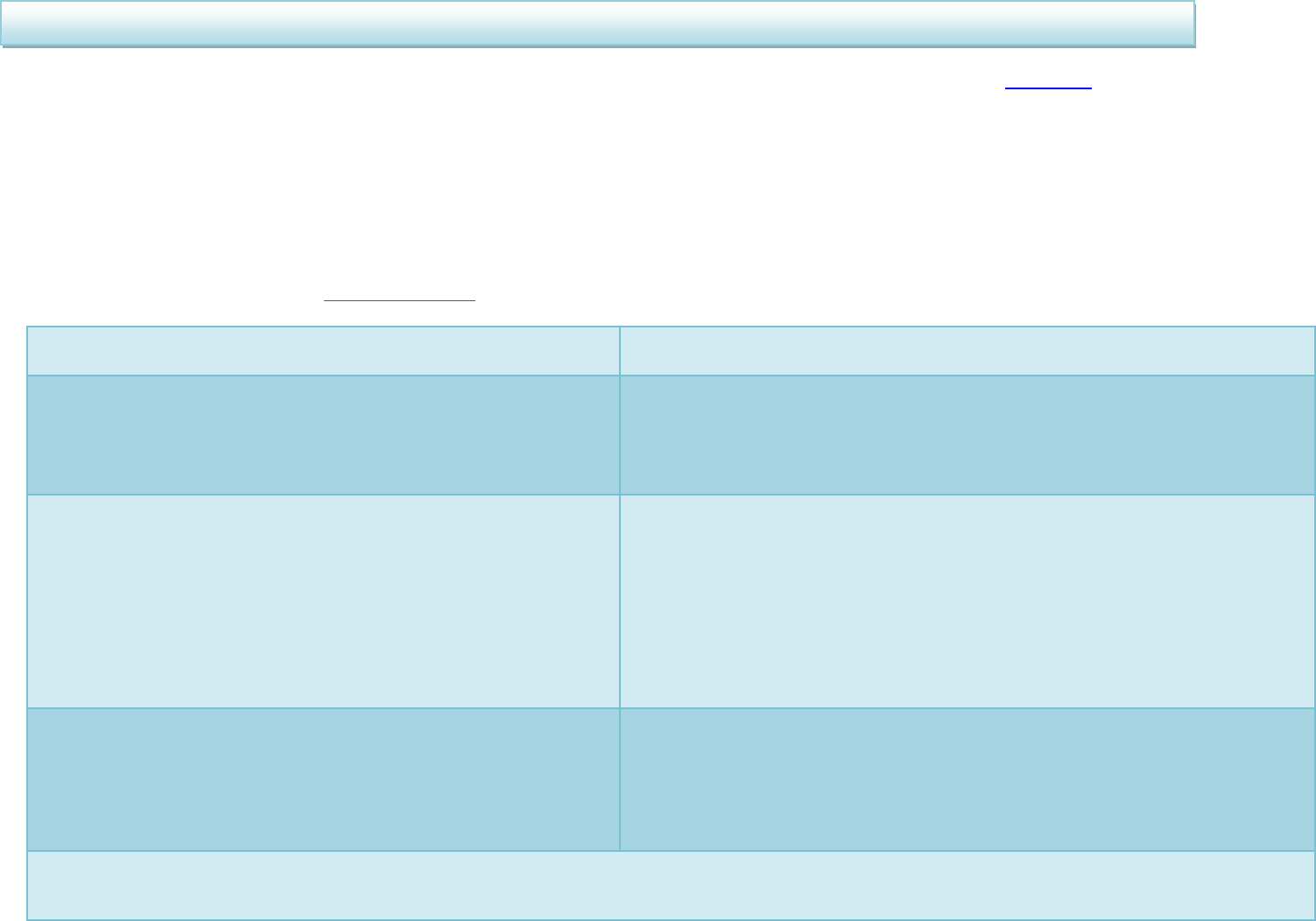
When should I treat Helicobacter pylori?
H pylori Positive
H pylori Negative
ASYMPTOMATIC post-HP
treatment
Treat H pylori
If H pylori negative treat as functional dyspepsia.
Step down to lowest dose of PPI or H
2
RA needed
to control symptoms. Review annually, including
PPI need.
Reassure, as
negative
predictive value
(NPV) of all tests
is >95%
Only retest for HP if DU, GU,
family history of cancer,
MALToma or if test was
performed within two weeks of
PPI or four weeks of antibiotics
When should I test for Helicobacter pylori (HP)?
When is a test for Helicobacter pylori not required?
• Eradication therapy is much more likely to succeed if the patient fully understands the reason for their treatment and is given full information and counselling to
encourage excellent adherence.
• Macrolide and quinolone resistance is an important risk factor for treatment failure. Metronidazole or tetracycline and amoxicillin resistance is less important.
• To reduce the emergence of resistance and Clostridioides difficile infection (CDI), avoid levofloxacin regimes unless no other options available.
• Doses detailed below assume non pregnant adults with normal renal and hepatic function.
• If post gastro-duodenal bleed, only start HP treatment when patient can take oral medication.
• If on intravenous antibiotics for concurrent illness which adhere to the 1
st
line drug choices below, the total IV/PO antibiotic duration should be 7 days.
• If diarrhoea develops, consider CDI and review need for treatment
*PPI regimes as per NHS Tayside formulary/PHE 2019 (omeprazole 20mg – 40mg bd or lansoprazole 30mg bd for 7 days)
**Consider quinolone warnings and interactions and prolonged QT with clarithromycin
NO PENICILLIN ALLERGY
PENICILLIN ALLERGY
FIRST LINE: 7 days
PPI bd*
PLUS amoxicillin 1g bd
PLUS either metronidazole 400mg bd
OR clarithromycin 500mg bd**
FIRST LINE: 7 days
PPI bd*
PLUS metronidazole 400mg bd
PLUS clarithromycin 500mg bd**
ONGOING SYMPTOMS after first line – SECOND LINE: 7 days
PPI bd*
PLUS amoxicillin 1g bd
PLUS second antibiotic not used in first line,
either clarithromycin 500mg bd** or metronidazole 400mg bd
FIRST LINE WITH PREVIOUS MACROLIDE EXPOSURE (in last 12 months) OR SECOND
LINE WITH PREVIOUS QUINOLONE EXPOSURE (in last 12 months) : 7 days
PPI bd*
PLUS bismuth subsalicylate 525mg qds
(or if not available consider tripotassium dicitratobismuthate 240mg qds (unlicensed)
– please note there is no file on vision for this product so it should be prescribed on a
paper GP 10 paper form and documented in patient journal)
PLUS tetracycline hydrochloride 500mg qds
PLUS metronidazole 400mg bd
ONGOING SYMPTOMS AFTER FIRST LINE AND PREVIOUS EXPOSURE TO
METRONIDAZOLE AND CLARITHROMYCIN – SECOND LINE: 7 days
PPI bd*
PLUS amoxicillin 1g bd
PLUS tetracycline 500mg qds OR
levofloxacin** 250mg bd (if tetracycline unsuitable)
ONGOING SYMTOMS AFTER FIRST LINE AND NO PREVIOUS EXPOSURE TO
LEVOFLOXACIN: 7 days
PPI bd*
PLUS metronidazole 400mg bd
PLUS levofloxacin**250mg bd
THIRD LINE: Only offer longer duration or third line therapy on advice from specialist
How should I treat Helicobacter pylori?
•
• Re-testing after eradication should not routinely be offered – 64% of patients with functional dyspepsia will have recurrent symptoms
• Offer if:
o Compliance poor, or high local resistance rates
o Persistent symptoms and HP test performed within 2 weeks of taking PPI, or within 4 weeks of taking antibiotics
o Patients with an associated peptic ulcer, after resection of an early gastric carcinoma or MALT lymphoma
o Patients requiring aspirin, where PPI is not co-prescribed
o Patients with severe persistent or recurrent symptoms, particularly if not typical of GORD
• Wait at least 4 weeks (ideally 8 weeks) after treatment. If acid suppression needed use H
2
RA
• Use second line treatment if test remains positive
(??
• Reassess need for eradication
• In patients with GORD or non-ulcer dyspepsia, with no family history of cancer or peptic ulcer disease, a maintenance PPI may be appropriate
• Patients in whom the choice of antibiotic is reduced due to hypersensitivity
• Patients who have received two courses of eradication treatment and remain HP positive
References:
Public Health England. Test and treat for Helicobacter pylori (HP) in dyspepsia. Quick reference guide for primary care: For consultation and local
adaptation. Updated Feb 2019.
NICE CG184. Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. Updated November 2019.
O’Connor A et al. Treatment of Helicobacter pylori in infection 2010. Helicobacter 2010 Sept;15 Suppl 1:46-52.
Tayside Area Formulary
ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212-238.
Helicobacter pylori Antibiotic Resistance in the United Stat... : Official journal of the American College of Gastroenterology | ACG (lww.com) May 2022
When should I retest for Helicobacter pylori?
What should I do in eradication failure?
What should I refer for endoscopy, culture and susceptibility testing?

HO, J et al. Helicobacter pylori Antibiotic Resistance in the United States between 2011-2021: A Systematic Review and Meta-analysis. American Journal of
Gastroenterology May 2022.
Approved by AMG June 2022
Review date June 2025
