EpiTuub
Fecal H. Pylori Antigen Rapid Test Kit - Instructions for Fecal Sample Collection
Qualitative detection of H. Pylori antigen in human feces.
Version 6
READ ALL THE INFORMATION IN THIS LEAFLET BEFORE SAMPLING
Store at 2-8°C. Do not freeze. Keep out of reach of children. For in-vitro diagnostic use. Not to be taken internally. Not to be sampled directly from anus.
If you have any questions, please contact your physician or laboratory staff or call Epitope Diagnostics at 858-693-7877 from 8:00 a.m. to 5:00 p.m. PST
Manufactured by Epitope Diagnostics, Inc. San Diego CA 92121, USA (V6/2019-08) Page 1 of 3 US Patent: 7,780,915
MDSS GmbH
Schiffgraben 41
30175 Hannover
Germany
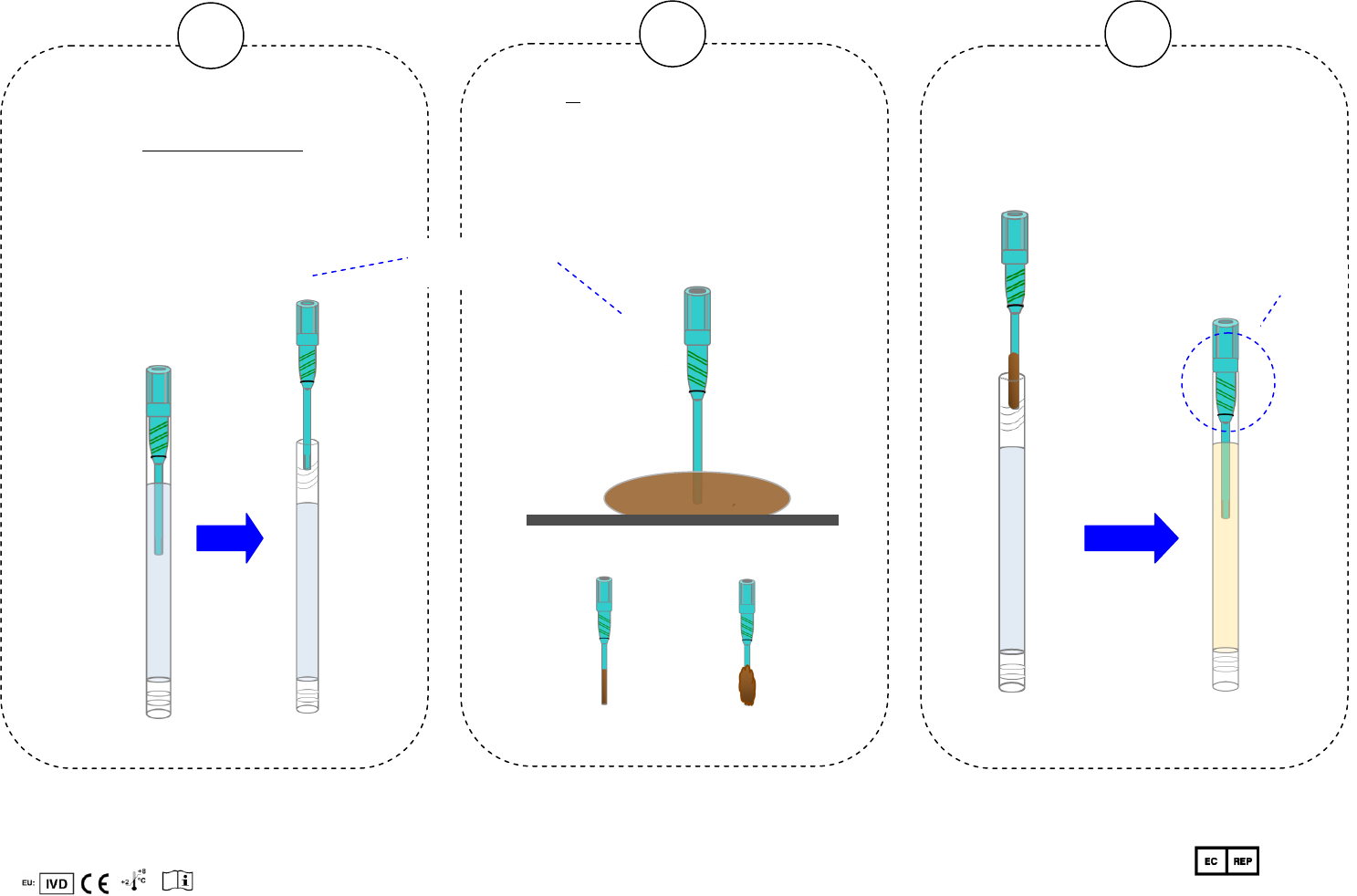
1. Collect a stool sample using the enclosed stool
sampling paper:
a. Clean the bowl and flush the toilet two times. Unfold
and lay the Sample Collection Paper directly on
top of the water in the toilet bowl (the paper should
float above the water).
b. After bowel movement, take the sampling tube
and unscrew the sampling lid, keeping the
sampling tube in a vertical position to prevent
loss of solution.
1. Hold the sampling lid by the Thumb Grip.
2. Use the tip of the sampling lid to collect a small
amount of fecal sample at two or more sites. Only
take the fecal sample that sticks to the sampling lid
tip (never intentionally place any separate piece of
fecal sample into the tube). The total amount of stool
collected should be less than one grain of cooked
rice. For liquid stool, collect 0.1mL into the sampling
tube.
1. Insert and screw the sampling lid back into the
sampling tube in a vertical position. Do not spill
any solution from the tube.
2. Tightly seal the lid with the tube.
3. Flush toilet.
1
2
3
Stool
Thumb Grip
Just right Too much
Sampling lid
Sampling
tube
Tightly seal here
Stool
FOR REFERENCE USE ONLY

EpiTuub® Fecal H. pylori Antigen Rapid Test Kit - Instructions for Test Procedures
Qualitative detection of H. Pylori antigen in human feces.
READ ALL THE INFORMATION IN THIS INSERT BEFORE TESTING
Store at 2-8°C. Do not freeze. Keep out of reach of children. For in-vitro diagnostic use. Not to be taken internally. Not to be sampled directly from anus.
If you have any questions, call customer information staff of Epitope Diagnostics at 1-858-693-7877, 8:00 a.m. to 5:00 p.m. PST.
Manufactured by Epitope Diagnostics, Inc. 7110 Carroll Rd, San Diego, CA 92121, USA (V6/2019-06) Page 2 of 3 US Patent: 7,780,915
MDSS GmbH
Schiffgraben 41
30175 Hannover
Germany
1. Shake sampling tube to dissolve the stool into
the solution.
2. Turn the sampling tube upside down
vertically.
3. Remove the test strip from foil pouch.
1. Insert and screw the test strip in a vertical
position into the sampling tube by breaking
the bottom seal of the sampling tube.
2. Allow the solution to flow into the bottom space
of test strip, keeping the device in a vertical
position.
3. You may soon see a red fluid moving across
the white area of the test strip. Read test result
after 5 minutes.
A
B
Sampling tube
Test Strip Tube
1. Negative
2. Positive
3. Invalid
Result
1. Tightly sealed
2. Solution reaches the test strip
For In-Vitro Diagnostic Use
Catalog Number: KT929 (30T/Kit)
KT929.10 (10T/Kit)
INTENDED USE
This H. pylori antigen test kit is intended for the direct qualitative detection
of the presence of H. pylori antigen in patient fecal samples. The test
might be used as an aid for detecting patients with acute and chronic
gastroenteritis infected with H. pylori.
SUMMARY OF PHYSIOLOGY
Helicobacter pylori (H. pylori) is a helical shaped gram-negative, about 3
micrometres long with a diameter of about 0.5 micrometre,
microaerophilic bacterium that infects various areas of the stomach and
duodenum. Many cases of peptic ulcers, gastritis, duodenitis, and cancers
are caused by H. pylori infections. However, many who are infected do
not show any symptoms of the disease. H. pylori is a contagious
bacterium. Many researchers think that H. pylori is transmitted orally by
means of fecal matter through the ingestion of waste tainted food or
water.
Diagnosis of gastroenteritis with H. pylori infection can be established
based on the detection of the bacteria specific antigen by a specific
immunoassay methods. The fecal H. pylori antigen test may also have
significant clinical tracking value in monitoring the effectiveness of
treatment and the recurrence of the infection in comparison to serum H.
pylori antibody test.
ASSAY PRINCIPLE
The EpiTuub® H. pylori Rapid Test employs dye-conjugated monoclonal
antibody against H. pylori antigen, and solid-phase/membrane coated
specific anti-H. pylori monoclonal antibody. In this test the specimen is
first treated with an extraction solution to extract H. pylori antigens from
the stool. Following extraction, the only step required is to screw the H.
pylori test strip tube into the sample collection tube. As the sample
extraction flows upward through chamber and reaches the test strip, the
colored particles migrate. In the case of a positive result, the specific
antibody present on the membrane will capture the colored particles.
Different colored lines will be visible, depending upon the bacteria content
of the sample. These lines, after 5 minutes of incubation at room
temperature, are used to interpret the result.
REAGENTS: Preparation and Storage
1. Fecal specimen collection device (30205): containing sampling tube,
sampling lid and pre-added extraction solution in the sampling tube. This
device should be stored at 2 to 8°C. Do not freeze.
FOR REFERENCE USE ONLY
EpiTuub
Fecal H. pylori Antigen Rapid Test Kit - Instructions for Test Procedures
Qualitative detection of H. pylori antigen in human feces.
Manufactured by Epitope Diagnostics, Inc. San Diego CA 92121, USA pAGE 3
(V6/2019-08) Page 3 PAGE 3 pPage 3 US Patent: 7,780,915
n
LIMITATION OF THE PROCEDURE
1. The test should be used only for the detection of H. pylori
antigen in fecal samples.
2. The test is qualitative, and no quantitative interpretation
should be made with respect to the intensity of the positive line,
when reporting the result.
3. Two hundred samples were evaluated to assure the correct
performance of the test. The correlation of the results with other
techniques (ELISA) was satisfactory. However, interferences in
the performance of the tests should not be excluded.
4. As with all diagnostic tests, the definitive clinical diagnosis
must not be based on the result of a single test, but should only
be made by the physician after all clinical and laboratory
findings have been evaluated. EpiTuub
TM
Fecal H. pylori
antigen test is designed for the aid of clinical diagnosis and
should not replace other diagnostic procedures.
PERFORMANCE CHARACTERISTICS
Sensitivity
Detection limit: A culture of H. pylori bacteria was sonicated,
centrifuged and its protein concentration was determined. This
reference antigen preparation of H. pylori was diluted in 0.01M
PBS-BSA buffer and tested with this kit according to the above
described test procedures. The detection limit of H. pylori is
about 4 – 8 ng/ml.
Specificity
The evaluation was performed by comparison this rapid test
with an commercial H. pylori antigen ELISA kit. The detection of
H. pylori showed 95% of concordance with the ELISA.
The monoclonal antibody used in this rapid test regognises
epitopes present in the antigen found in stool of patients, as
well as in preparations from the bacteria cultures in vitro.
Sonicated H. pylori extract from different commercial samples
reacts with this H. pylori antigen rapid test.
The possibility for interference of human anti-mouse antibodies
(HAMA) or high levels of rF in the stool sample have not been
evaluated.
REFERENCES
1. Yang HR, Seo JK. Helicobacter pylori Stool Antigen (HpSA) Tests in Children Before and
After Eradication Therapy: Comparison of Rapid Immunochromatographic Assay and HpSA
ELISA. Dig Dis Sci. 2007 Dec 13;
2. Wu DC, Wu IC, Wang SW, Lu CY, Ke HL, Yuan SS, Wang YY, Chang WH, Wang TE, Bair
MJ, Kuo FC. Comparison of stool enzyme immunoassay and immunochromatographic method
for detecting Helicobacter pylori antigens before and after eradication. Diagn Microbiol Infect
Dis. 2006 Dec;56(4):373-8.
3. Kato S, Ozawa K, Okuda M, Fujisawa T, Kagimoto S, Konno M, Maisawa S, Iinuma K.
Accuracy of the stool antigen test for the diagnosis of childhood Helicobacter pylori infection: a
multicenter Japanese study. Am J Gastroenterol. 2003 Feb;98(2):296-300.
2. Test strip tube (30197): one dipstick for the H. pylori test is
assembled in a transparent housing and sealed in a foil
pouch with desiccant. It should remain in its original sealed
pouch until ready for use. The test strip should be stored
at 2 to 8ºC. Do not freeze.
3. Instruction for use.
MATERIALS REQUIRED BUT NOT SUPPLIED
1. Timer or clock
PRECAUTIONS
1. For in-vitro diagnostic use only. Not to be taken internally.
2. Do not use product beyond the expiration date.
3. Handle all specimens as potentially infectious.
4. Do not reuse the test.
PATIENT PREPARATION
1. Dietary restrictions are not necessary.
SPECIMEN COLLECTION
1. Stool specimens can be collected at any time of the day.
2. Collect a random sample of feces in a clean, dry cup or
toilet paper or as indicated in the Figure 1.
3. Unscrew the sampling lid and keep the sampling tube in a
vertical position to prevent the loss of any extraction
solution.
4. Insert and twist the tip of the sampling lid into the stool
specimen at two or more different sites (Figure 2).
5. Collect fecal sample that is stuck to the surface of the
sampling lid. The total amount of stool sample should be
less than one grain of cooked rice. Do not intentionally
collect any separate and large pieces of fecal sample into
the tube.
6. Replace the sampling lid into the tube and secure tightly
(Figure 3).
7. The specimen is ready for testing, transportation or
storage. It can be stored at 2-8ºC for up to 21 days and at
room temperature for up to 14 days.
TEST PROCEDURE
1. Bring the sealed foil pouch test strips and collected
specimens to room temperature.
2. Shake the sampling tube vigorously to ensure a good liquid
suspension.
3. Position the sampling tube upside down vertically and let it
settle for about 1 minute.
4. Remove the test strip from the sealed foil pouch.
5. Screw the test strip tube into the sampling tube by breaking the
bottom seal of the sampling tube. Secure tightly! (Figure A)
6. Allow the solution to flow into the bottom space of the test strip
and keeping the device in a vertical position.
7. Read test result at 5 minutes. Do not interpret test result after 10
minutes.
PROCEDURAL NOTES
1. After the test strip tube is screwed completely into the sampling
tube, you should see a minimum 5 mm extraction buffer liquid in
the bottom of the strip tube.
2. You should see liquid migrating across the membrane area
right after the screw in process. If not, take the tube and tap
against the table several times, and the migration of the liquid
should be observed.
INTERPRETATION OF RESULTS
Positive:
If two red/pink colored bands are visible within 5 minutes, the
test result is positive and valid (Figure B).
Negative:
If test area has no red/pink colored band and the control area
displays a red/pink colored band, the test result is negative
(Figure B).
Invalid:
If a colored band does not form in the control area regardless of
there being any band in the test area, the test result is invalid
(Figure B) and needs to be retested.
QUALITY CONTROL
Good laboratory practices recommend the use of appropriate
controls. There are two types of controls for the EpiTuub®
H.pylori
test, the internal procedural control and external controls.
1. Internal procedural control: Each EpiTuub® H. pylori test has
a built-in procedural control. It will appear if the test has been
performed correctly, sample wicking has occurred and the
reagents are reactive. It does not ensure that the test line
antibody is accurately detecting the presence or absence of H.
pylori in the test fecal sample.
2. External controls: It is recommended to use external positive
controls. The external positive controls are not provided with this
kit, but are commercially available from Epitope Diagnostics.
External controls are used to assure that the test line antibody is
reactive. However, external controls will not detect an error in
performing the patient sample test procedure. It is
recommended that the external control be tested once per kit.
Follow local, state, and federal guidelines for running quality control.
FOR REFERENCE USE ONLY
